|
|
|
|
|
HONG KONG, June 21, 2022 - (ACN Newswire) - SinoMab BioScience Limited ("SinoMab" or the "Company", together with its subsidiaries, the "Group", stock code: 3681.HK), a Hong Kong-listed biopharmaceutical company dedicated to the research, development, manufacturing and commercialization of innovative therapeutics for the treatment of immunological diseases, primarily mAb-based biologics, today announced that on 10 June 2022, the mechanism of action of its flagship product SM03 (Suciraslimab) is successfully published in the Journal of Immunology, a reputable journal on immunology in the US (link to the paper: https://www.jimmunol.org/content/early/2022/06/10/jimmunol.2100820).
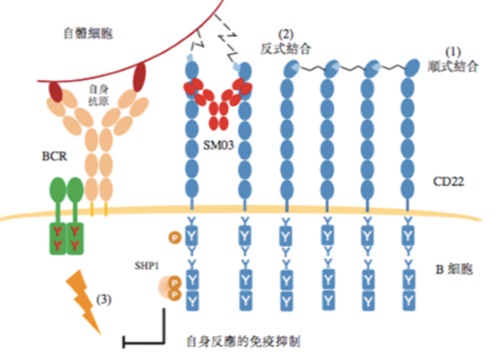 | | Figure: Mechanism of Action of SM03 (Suciraslimab) |
The Journal of Immunology (the JI), founded in 1916 and managed and published by the American Association of Immunologists, is the world's leading peer-reviewed journal in the field of immunology, with a 5-year impact factor of 6.029 (Journal Citation Reports 2020), and publishes the latest immune-related findings in basic and clinical research in the field of immunology. It also publishes the latest research papers in the field of immunology, including cellular immunology, immunochemistry and molecular immunology, immunogenetics and immunomodulation, immunopathology and clinical immunology, immunopharmacology, microbial immunology, oncology and transplantation.
The Company's flagship product SM03 (Suciraslimab) is a potential global first-in-target mAb against CD22 for the treatment of rheumatoid arthritis (RA). The patient enrollment for the Phase III clinical trial in China has been completed by the end of 2021, with a total of 530 subjects recruited. The trial is a multi-centre clinical study led by Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences, with clinical centres in 44 hospitals including Gulou Hospital of Nanjing University School of Medicine, the Second Hospital of Harbin Medical University and the First Hospital of Shanxi Medical University. The Company expects to lock the database at the end of June this year, read out the results of Phase III clinical trial data in early August and will have a biologics license application (BLA) in 2023. In addition, the development of Suciraslimab has also received support from the 863 Program, the "12th Five-Year Plan" and the "13th Five-Year Plan" for the development of major new drugs, as well as the green channel for "prioritized review and approval" by the Center for Drug Evaluation (CDE). Previously, a phase II clinical trial led by Peking Union Medical College Hospital has demonstrated the efficacy and safety of Suciraslimab for the treatment of RA.
The Company's flagship product Suciraslimab is a potential global first-in-target mAb against CD22 for the treatment of rheumatoid arthritis. The core competitive advantage of this product over the standard therapies currently on the market is its novel and unique mechanism of action, which ensures that Suciraslimab is comparable to other products in terms of efficacy while having a significant advantage in terms of safety, which is a major concern for patients on long-term medication for autoimmune diseases.
Under normal operation of the human immune system, the B-cell receptor (BCR) pathway would be activated and create strong signals in response to foreign ("non-self") antigens and trigger a series of B-cell immune responses. To differentiate from our "self" antigens, our body would recruit molecules, like SHP-1, to inhibit or reduce BCR-induced signaling, thereby suppressing B-cell immune responses. We suggested that the recruitment of these molecules such as SHP-1 could be achieved by conversion of cis-binding CD22 to trans-binding CD22, thus suppressing relevant immune responses.
Due to aging or genetic predisposition, patients with autoimmune diseases such as RA are unable to convert cis-binding CD22 to trans-binding CD22, and are thus unable to recruit immunosuppressive molecules such as SHP-1 to inhibit or reduce the transmission of antigenic stimuli from B-cell receptors to B-cells, which then generate a series of immune responses, such as the secretion of large amounts of antibodies to attack autoantigens. Our flagship product, Suciraslimab, can facilitate the conversion of cis-binding CD22 to trans-binding CD22, forming a stable CD22 trans-binding structure, thereby restoring the tolerance of B-cells to autoantigens and inhibiting a series of relevant immune responses by B cells to attack the body.
Historical data shows that Suciraslimab has a significant safety advantage compared to other products with comparable efficacy currently available in the market. Existing RA therapies include traditional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) such as methotrexate (MTX), biologic DMARDs (bDMARDs) targeting tumour necrosis factor (TNF), interleukin (IL)-6 receptors and other targets, as well as targeted synthetic DMARDs (tsDMARDs) such as Janus kinase (JAK) inhibitors and other small molecule drugs, all of which have played an important role in the remission of RA patients. However, most of the mechanisms of action of existing RA therapies will result in the depletion or death of B cells, which can have a series of side effects on the human autoimmune system, thus posing a long-term risk for patients with autoimmune diseases who require long-term medication, as the weakening of the immune system will naturally lead to the development of other debilitating diseases. In contrast, the mechanism of action of Suciraslimab is very different from that of existing therapies on the market. We only inhibit the B-cell-related immune response by altering the binding of CD22 and recruiting-related inhibitory molecules. Suciraslimab only inhibits the autoimmune response by regulating the function of B-cells and does not damage the B-cells and does not affect the normal function of B-cells in the immune system. Therefore, Suciraslimab has a significant safety advantage over other products currently available on the market.
The autoimmune disease drug market has been touted as the next gold mine after oncology drugs. As the "king of drugs" in the world, Humira has topped the global drug ranking for eight years with sales of US$19.832 billion in 2020, making other drugs "bow down". Globally, pharmaceutical giants are focusing on the research and development of drugs in the rheumatic immune field, with the most prominent ones including Pfizer, Novartis, Johnson & Johnson and AbbVie, all with more than a dozen products in development or marketed in their respective pipelines. The global autoimmune disease drugs market is valued at US$113.7 billion in 2018 and is expected to reach US$152.3 billion by 2023 at a CAGR of 6.0%. The market for autoimmune disease drugs in China is expected to grow from RMB13.4 billion in 2018 to RMB37.7 billion in 2030 at a CAGR of 23%. Driven by the global trend of innovation, R&D in the rheumatic immune field in China is also gaining momentum. As a leading pharmaceutical innovator in China, Hengrui Pharma has also adopted a strategy of early development, target optimization and expansion of indications in the rheumatic immune field, aiming to fully capitalize on the rapid development of the autoimmune disease market in China. This indicates that SinoMab's mission and vision to become a global leader in innovative therapies for immune and other debilitating diseases are in line with the development trend of the entire pharmaceutical industry. In the foreseeable future, it is believed that SinoMab will help more and more patients suffering from autoimmune diseases and become a leading global biopharmaceutical company focusing on innovative therapies for autoimmune diseases.
In addition to its flagship product Suciraslimab, the Company's core product SN1011 has an expanding scope of indications and continues to receive regulatory approval for new drug applications in China and abroad, and also expects to rapidly initiate Phase II clinical trials for pemphigus vulgaris (PV), multiple sclerosis (MS), systemic lupus erythematosus (SLE) and neuromyelitis optica spectrum disorder (NMOSD) this year. SinoMab also dosed first healthy subject in the Phase I clinical trial of its major product SM17 in the U.S. in June 2022. By targeting upstream mediators of the Th2 inflammatory cascade, SM17 is able to control inflammatory airway diseases such as asthma caused by the immune cascade at a relatively early stage and is expected to benefit a large number of patients with severe uncontrolled asthma by satisfying unmet medical needs.
About SinoMab BioScience Limited
SinoMab BioScience Limited (stock code: 3681.HK) is dedicated to the research, development, manufacturing and commercialization of therapeutics for the treatment of immunological diseases. The Company's flagship product SM03 is a potential global first-in-target mAb against CD22 for the treatment of rheumatoid arthritis (RA) and is currently in Phase III clinical trial for RA in China, which has been recognized as one of the significant special projects of Significant New Drugs Development of the Twelfth Five-Year Plan Period and the Thirteenth Five-Year Plan Period. In addition, the Company possesses other potential first-in-target and first-in-class drug candidates, some of which are already in the clinical stage, with their indications covering rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), pemphigus vulgaris (PV), non-Hodgkin's lymphoma (NHL), asthma, and other diseases with major unmet clinical needs.
Topic: Press release summary
Source: SinoMab BioScience Limited
Sectors: BioTech, Healthcare & Pharm
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|
|

|
|
|
|