|
| Wednesday, 10 June 2015, 15:20 HKT/SGT | |
| |  | |
Source: Nanobiotix | |
|
|
|
|
| Evaluation of NBTXR3 safety profile by an independent committee and enlargement of targeted population |
PARIS, FRANCE, June 10, 2015 - (ACN Newswire) - NANOBIOTIX (Euronext: NANO - ISIN: FR0011341205), a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer, announces positive preliminary safety results for its lead NanoXray product NBTXR3, in its head and neck cancer Phase I/II clinical trial.
 | | Nanobiotix Reports Positive Preliminary Results in Head and Neck Cancer Phase I/II Clinical Trial with NBTXR3 |
- Intermediate results show the feasibility of the injection of NBTXR3 and a good safety profile of the product for this indication
- Good safety results allowing the enlargement of the targeted population to patients treated in combination with radiotherapy plus chemotherapy
- One step further for use and transferability of NBTXR3 therapeutic approach in different types of tumors
A significant proportion of Head and Neck carcinomas in the western world are found in the oral cavity. The oropharynx is the posterior continuation of the oral cavity and connects with the nasopharynx (above) and laryngopharynx (below). It is also a frequent site of primary Head and Neck cancers. These structures play a crucial role in swallowing, breath and speech. Locally advanced oropharyngeal cancers can obstruct the air flow or infiltrate muscles or nerves, which significantly disturb local functions.
This trial is an open-label non-randomized, dose escalation study. The primary objectives are the evaluation of NBTXR3 safety and tolerability. The secondary objectives of this trial include: assessment of the tumor Response Rate and complete Response Rate by MRI; evaluation of local and general Progression Free Survival of NBTXR3 and the assessment of the feasibility of local administration.
Positive preliminary results
An independent safety committee of experts just gave a positive assessment of the safety profile of NBTXR3 injection, based on the 2 first dose levels preliminary results of the phase I/II of Head and Neck clinical trial.
The intermediate results show the feasibility of NBTXR3 injection at the 2 first dose levels evaluated. Volumes equivalent to 5% and 10% of the tumor size have been tested by intra tumoral injection. For the record, 10% is the recommended volume in the Soft Tissue Sarcoma indication, currently evaluated in a study for registration (phase II/III).
These results also confirm NBTXR3 good safety profile, as no Serious Adverse Events related to the product have been observed and as the product appears to stay within the tumor with no leakage in the surrounding healthy tissues.
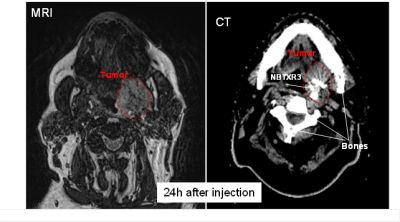
Figures: Patient treated with NBTXR3; MRI (visualization of the tumor) and CT Scan (visualization of the nanoparticles) taken both 24h after injection showing the presence of the product within the tumor.
http://www.acnnewswire.com/topimg/Low_nano150610.jpg
In this trial, patients are being assessed particularly on their tolerance to the product. Exploratory efficacy endpoints will be evaluated and shown at the end of this clinical trial.
Expansion of the Head and Neck trial to address a larger population
The first part of this phase I/II clinical trial has targeted frail and elderly patients who cannot receive chemotherapy with radiotherapy and need new innovative treatments. These patients represent approximately 11% of the Head and Neck cancer population.
The positive safety results on the first levels and additional preclinical data that have been generated, allow to enlarge the scope of patients that could be targeted within the Head and Neck indication.
As a consequence, Nanobiotix plans to include in the continuation of this Phase I/II clinical trial, patients that are receiving Cisplatin in combination with radiotherapy, this combinaison is the reference treatment for Head and Neck cancers.
In this population, locoregional recurrence remains the dominant cause of treatment failure. There is a strong rationale for the use of NBTXR3 to improve locoregional control through radiotherapy enhancement for successful Head and Neck cancer treatment.
Studies in animals testing NBTXR3 with radiotherapy and Cisplatin have shown antitumor synergy with a very good tolerance. These data conjointly with the good safety profile observed in the treated patients have determined this more ambitious strategy.
Currently, the combinasion of Cisplatin with radiotherapy is given to 35-40% of patients with Head and Neck carcinomas. The inclusion of these patients would magnify the total potential treatable population for NBTXR3 in this indication.
One step further in the transferability of the NBTXR3 therapeutic approach
Soft Tissue Sarcoma is the most advanced indication for the NBTXR3 product. Currently, it is studied in a registration phase (II/III). CE mark is anticipated end 2016. The phase I/II of this trial showed successful feasibility and safety of the injection of NBTXR3 product with varying volumes and in different sarcoma subtypes.
The similarity of results so far in term of product behavior found in the Head and Neck study presents additional rationale for transferability of the product to many indications.
Elsa Borghi, CMO of Nanobiotix commented: "These results represent critical advances for the global clinical development of NBTXR3. The principles of translational research are even stronger because the good local safety observed so far supports the planned initiation in other indications such as prostate cancer for example. In fact, the demonstration of the permanency of NBTXR3 within the tumor without leakage to the healthy tissues reinforces the relevance of using the NBTXR3 radioenhancer to solve limitations of radiotherapy in multiple clinical applications."
About Phase I/II clinical trials of NBTXR3 in Head and Neck cancer
The phase I trial is an open-label non-randomized, dose escalation study of safety and tolerability evaluation of NBTXR3. The product is implanted by intra-arterial (IA) or intra-tumor (IT) injection, and activated by high precision radiation therapy (intensity-modulated radiation therapy - IMRT) delivered as per current medical practice.
Population for part one of the trial are patients with locally advanced squamous cell carcinoma of the oral cavity or oropharynx constitute the targeted population: frail and elderly patients.
Expected enlargement of patient population (in addition to the first one) for continuation of the trial: patients receiving radiotherapy in combination with chemotherapy.
Based on the observed safety, the recommended doses for further evaluation of NBTXR3 with radiotherapy will be selected.
The secondary objectives of the study include assessment of the tumor Response Rate and complete Response Rate by MRI, and the evaluation of local and general Progression Free Survival of NBTXR3. Furthermore, the feasibility of local administration, either intra-tumor or intra-arterial injection of NBTXR3, then activated by radiotherapy will be evaluated.
About head and neck cancer
Head and neck cancer represents a group of aggressive cancers that appears in the mouth, the nose, the sinuses and at the top of the aerodigestive tract. This type of cancer is highly curable if detected early. In more advanced tumors, the association of surgery, chemotherapy and radiation therapy constitute the current approaches of treatment. However, surgery has detrimental effects on the patient's function, in terms of swallowing, breathing or speech - and cosmetic appearance.
Head and neck cancers are a major concern of public health in some European countries and across Asia. There is an immediate need for innovative therapies in these diseases. NBTXR3 may significantly help to improve the quality of life in this patient population.
About NANOBIOTIX www.nanobiotix.com
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer. The company's first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy with a view to provide a new, more efficient treatment for cancer patients. NanoXray products are compatible with current radiotherapy treatments and are meant to treat potentially a wide variety of cancers including Soft Tissue Sarcoma, Head and Neck Cancer, Liver Cancers, Prostate Cancer, Breast Cancer, Glioblastoma, etc., via multiple routes of administration.
Nanobiotix's lead product NBTXR3, based on NanoXray, is currently under clinical development for Soft Tissue Sarcoma and locally advanced Head and Neck Cancer. The company has partnered with PharmaEngine for clinical development and commercialization of NBTXR3 in Asia.
Nanobiotix is listed on the regulated market of Euronext in Paris (ISIN: FR0011341205, Euronext ticker: NANO, Bloomberg: NANO: FP). The company Head Quarter is based in Paris, France. Affiliate in Cambridge United States.
Contact
Nanobiotix
Sarah Gaubert
Head of Communication and Public Affairs
+33 (0)1 40 26 07 55
contact@nanobiotix.com
Media relations
France - NewCap
Annie-Florence Loyer
+33 (0)6 88 20 35 59
afloyer@newcap.fr
Outside France - Instinctif Partners
Melanie Toyne Sewell / Jayne Crook
+44 (0) 207 457 2020
nanobiotix@instinctif.com
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the 2014 annual financial report of (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country.
###
This announcement is distributed by NASDAQ OMX Corporate Solutions on behalf of NASDAQ OMX Corporate Solutions clients.
The issuer of this announcement warrants that they are solely responsible for the content, accuracy and originality of the information contained therein.
Source: NANOBIOTIX via Globenewswire
Topic: Research and development
Source: Nanobiotix
Sectors: Biometrics, Science & Research
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2025 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|
|

|
|
|
|