|
| Friday, 8 March 2013, 13:30 HKT/SGT | |
| |  | |
Source: A*STAR | |
|
|
|
|
| Patients with diseases such as diabetes suffer from painful wounds that take a long time to heal making them more susceptible to infections that could even lead to amputations. A*STAR's discovery paves the way for therapeutics to improve healing of such chronic wounds, which are a significant burden to patients. |
SINGAPORE, Mar 8, 2013 - (ACN Newswire) - Scientists from A*STAR's Institute of Medical Biology (IMB) have identified a molecular "switch" that controls the migration of skin cells necessary for wounds to close and heal. This is especially significant for diabetics and other patients who suffer from chronic wounds, wounds that do not heal or take years to do so, which are vulnerable to infections and could lead to amputations. This switch mechanism may hold the key to developing therapeutics that will reduce or prevent chronic wounds.
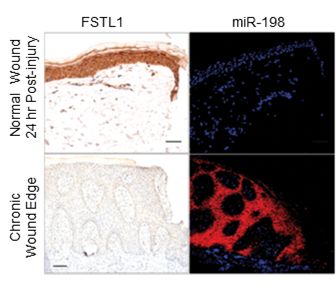 | | Expression of FSTL1 and miR-198 in normal wounds vs. chronic wounds |
The scientists discovered that a tiny "micro-RNA" molecule, called miR-198, controls several different processes that help wound healing, by keeping them switched off in healthy skin. When skin is wounded, the manufacture of miR-198 quickly stops and the levels of miR-198 drop, switching on many wound healing processes.
In the non-healing wounds of diabetics, miR-198 does not disappear and wound healing remains blocked. This therefore identifies miR-198 as a potential diagnostic biomarker for non-healing wounds. These findings were recently published in the prestigious journal Nature(1).
The research leading to this discovery was carried out in collaboration with A*STAR's Bioinformatics Institute (BII), National University Hospital (NUH), Singapore and Jnana Sanjeevini Diabetes Center, Bangalore, India.
Importance of this discovery
Chronic wounds in patients with diabetes are a major global health burden and the most common cause of lower extremity amputations. In Singapore, diabetes is the fifth most common medical condition diagnosed and one in nine people aged 18 to 69 has diabetes(2). Chronic wounds also tend to affect the elderly and disabled patients, especially those confined to a wheelchair or bed-bound. Unfortunately, chronic wounds are currently poorly understood and insufficiently treated.
Dr. Prabha Sampath(3), principal investigator at IMB and lead author of the paper, said, "Our research provides a comprehensive understanding of the mechanism of the wound healing process. Moving forward, we hope to translate this research into improved patient outcomes. We can now build on this research, to see how we can modulate the defective switch in chronic wounds by targeting miR-198 and its interacting molecules, to develop new strategies for treating chronic wounds."
Professor Birgitte Lane, Executive Director of IMB, said, "This switch appears to be an entirely new regulatory component in wound healing, and probably a very important one. Poor wound healing is a major healthcare burden, and this discovery is particularly timely in the face of aging populations and the sharp global rise in diabetes. The finding gives us a platform from which to develop therapies that could significantly reduce chronic wounds and improve healthcare."
An FSTL1-miR-198 molecular 'see-saw' switch
The information necessary to express microRNA-198 (miR-198) and follistatin-like 1 (FSTL1) protein are found in a single "message" produced by the cell. However, miR-198 and FSTL1 protein cannot be produced at the same time - it can only be one or the other. These two molecules also have opposite roles: miR-198 (found in unwounded skin) inhibits skin cell migration and wound healing, whereas FSTL1 protein (expressed after injury) promotes skin cell migration and wound healing. A regulatory switch dictates their expression, and hence controls the "see-saw" between inactive resting skin cells and the cell migration necessary for wound healing.
Dr. Sampath and her team showed that healthy unwounded skin contained high levels of miR-198 but no FSTL1 protein. They demonstrated that these high levels of miR-198 prevent skin cell migration by suppressing several genes, such as PLAU, LAMC2 and DIAPH1(4), which are needed for different aspects of the wound healing process. However upon injury, miR-198 is switched off in the wound by a signal from transforming growth factor Beta1 (TGF-Beta1). This allows FSTL1 to now be made instead, and the skin migration genes to be unblocked, promoting migration of skin cells into the wound area to drive skin wound healing.
The scientists further examined skin samples of chronic non-healing ulcer wounds from patients with diabetes mellitus. They observed that, unlike healthy skin that had been injured, there remained high levels of miR-198 (inhibiting skin cell migration and wound healing) and an absence of FSTL1 protein (promoting skin cell migration upon wounding), indicating that this "switch" is defective in chronic wounds.
Please see the full press release, with supporting images, at http://bit.ly/ZvGHwm .
Notes for Editor:
(1) The findings "'See-saw' expression of microRNA-198 and FSTL1 from a single transcript in wound healing" were published in the online issue of Nature on 10th February 2013. doi:10.1038/nature11890
(2) http://www.diabetes.org.sg/
(3) Dr. Sampath is the holder of a prestigious A*STAR Investigator Award (2007); she joined the IMB to set up her own research group in May 2008.
(4) PLAU, LAMC2 and DIAPH1 proteins promote keratinocyte migration
The research findings described in this news release can be found in Nature under the title "'See-saw' expression of microRNA-198 and FSTL1 from a single transcript in wound healing" by Gopinath M. Sundaram1,*, John E. A. Common[1],*, Felicia E. Gopal1, Satyanarayana Srikanta[2], Krishnaswamy Lakshman[2], Declan P. Lunny[1], Thiam C. Lim[3][4], Vivek Tanavde[1][5], E. Birgitte Lane[1][6][7] & Prabha Sampath[1][7]. Doi:10.1038/nature11890
[1] Institute of Medical Biology, A*STAR, Singapore
[2] Jnana Sanjeevini Diabetes Center, India
[3] Division of Plastic, Reconstructive & Aesthetic Surgery, NUHS, Singapore
[4] Department of Surgery, Yong Loo Lin School of Medicine, NUS, Singapore
[5] Bioinformatics Institute, A*STAR, Singapore
[6] Department of Pathology, Yong Loo Lin School of Medicine, NUS, Singapore
[7] Department of Biochemistry, Yong Loo Lin School of Medicine, NUS, Singapore
About the Institute of Medical Biology (IMB)
IMB is one of the Biomedical Sciences Institutes of the Agency for Science, Technology and Research (A*STAR). It was formed in 2007, the 7th and youngest of the BMRC Research Institutes, with a mission to study mechanisms of human disease in order to discover new and effective therapeutic strategies for improved quality of life. Since 2011, IMB also hosts the inter-research institute Skin Biology Cluster platform. IMB has 20 research teams of international excellence in stem cells, genetic diseases, cancer and skin and epithelial biology, and works closely with clinical collaborators to target the challenging interface between basic science and clinical medicine. Its growing portfolio of strategic research topics is targeted at translational research on the mechanisms of human diseases, with a cell-to-tissue emphasis that can help identify new therapeutic strategies for disease amelioration, cure and eradication. For more information about IMB, please visit www.imb.a-star.edu.sg .
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is the lead agency for fostering world-class scientific research and talent for a vibrant knowledge-based and innovation-driven Singapore. A*STAR oversees 14 biomedical sciences and physical sciences and engineering research institutes, and six consortia & centres, located in Biopolis and Fusionopolis as well as their immediate vicinity. A*STAR supports Singapore's key economic clusters by providing intellectual, human and industrial capital to its partners in industry. It also supports extramural research in the universities, and with other local and international partners. For more information about A*STAR, please visit www.a-star.edu.sg .
Contact:
Ong Siok Ming (Ms)
Senior Officer, Corporate Communications
Agency for Science, Technology and Research
Tel: +65 6826 6254, +65 9733 7434
Email: ong_siok_ming@a-star.edu.sg
Topic: Research and development
Source: A*STAR
Sectors: Science & Research, BioTech
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|
|

|
|
|
|