|
| Monday, 1 September 2014, 12:00 HKT/SGT | |
| |  | |
Source: A*STAR | |
|
|
|
|
| A*STAR scientists find new targets for modulating antibody response |
SINGAPORE, Sept 1, 2014 - (ACN Newswire) - Scientists from A*STAR's Bioprocessing Technology Institute (BTI) have uncovered the crucial role of two signalling molecules, DOK3 and SHP1, in the development and production of plasma cells. These discoveries, published in two prestigious journals PNAS and Nature Communications, advance the understanding of plasma cells and the antibody response, and may lead to optimisation of vaccine development and improved treatment for patients with autoimmune diseases such as lupus and tumours such as multiple myeloma[1].
 | | Understanding and Improving the Body's Fight Against Pathogens |
While they exist in small populations in humans, the large amounts of antibodies secreted by plasma cells make them key to the body's immune system and its ability to defend itself against pathogens, such as bacteria and viruses. Proper maintenance of a pool of plasma cells is also critical for the establishment of lifelong immunity elicited by vaccination.
Dysregulation of plasma cell production and maintenance could lead to autoimmune diseases and multiple myeloma. Autoimmune diseases occur when the immune system does not distinguish between healthy tissue and antigens, which are found in pathogens. This results in expansion of plasma cells which produce excessive amounts of antibodies leading to destruction of one's own healthy tissue. The discoveries by scientists in BTI's Immunology Group have improved understanding of the mechanism by which plasma cells are developed from a major class of white blood cells called B cells.
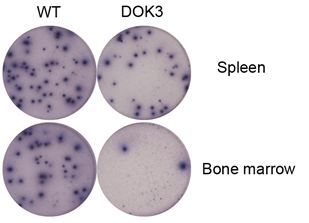
For the first time, the molecule DOK3 was found to play an important role in formation of plasma cells. While calcium signalling typically controls a wide range of cellular processes that allow cells to adapt to changing environments, it was found to inhibit the expression of the membrane proteins essential for plasma cell formation. These membrane proteins include PDL1 and PDL2, and represent some of the key targets for the development of immunotherapy by pharmaceutical companies. DOK3 was able to promote the production of plasma cells by reducing the effects of calcium signalling on these membrane proteins. The absence of DOK3 would thus result in defective plasma cell formation.
In another study, BTI scientists discovered the importance of SHP1 signalling to the long term survival of plasma cells. While the molecule SHP1 has a proven role in prevention of autoimmune diseases, it was found that the absence of SHP1 would result in the failure of plasma cells to migrate from the spleen where they are generated to the bone marrow, a survival niche where they are able to survive for much longer periods. This could result in a reduction of the body's immune response and thus, an increased susceptibility to infections and diseases. The scientists in this study also successfully rectified the defective immune response caused by an absence of SHP1 by applying antibody injections, which might advance the development of therapeutics. On the other hand, targeting SHP1 might be a strategy to treat multiple myeloma where the accumulation of cancerous plasma cells in the bone marrow survival niches is undesirable.
Findings hold potential for improved treatment
The discovery of these new targets for modulating the antibody response allows the development of novel therapeutic strategies for patients with autoimmune diseases and cancer. Understanding the mechanism that governs plasma cell differentiation is also critical for the optimal design of vaccines and adjuvants, which are added to vaccines to boost the body's immune response.
Prof Lam Kong Peng, Executive Director of BTI, said, "These findings allow better understanding of plasma cells and their role in the immune system. The identification of these targets not only paves the way for development of therapeutics for those with autoimmune diseases and multiple myeloma, but also impacts the development of immunological agents for combating infections."
[1] Multiple myeloma is a cancer of plasma cells that causes bone debilitating diseases. It is considered to be incurable but treatable.
Notes to Editor:
The research findings described in this media release can be found in:
1. Proceedings of the National Academy of Sciences of the United States of America (PNAS) Journal, under the title, "Adaptor protein DOK3 promotes plasma cell differentiation by regulating the expression of programme cell death 1 ligands" by Xijun Ou,1, Shengli Xu,1,2, Yan-Feng Li,1, Kong Peng Lam,1,2,3,4
Full text of the PNAS paper can be accessed online from: http://www.pnas.org/content/early/2014/07/22/1400539111.abstract
2. Nature Communications Journal, under the title, "Shp1 signalling is required to establish the long-lived bone marrow plasma cell pool" by Yan-Feng Li,1, Shengli Xu,1,2, Xijun Ou,1, Kong Peng Lam,1,2,3,4.
1 Immunology Group, A*STAR Bioprocessing Technology Institute, Singapore
2 Department of Physiology, NUS Yong Loo Lin School of Medicine, Singapore
3 Department of Microbiology, NUS Yong Loo Lin School of Medicine, Singapore
4 Department of Pediatrics, NUS Yong Loo Lin School of Medicine, Singapore
Full text of the Nature Communications paper can be accessed online from: http://www.nature.com/ncomms/2014/140630/ncomms5273/full/ncomms5273.html
About the Bioprocessing Technology Institute (BTI)
Bioprocessing Technology Institute (BTI) is a member of the Agency for Science, Technology and Research (A*STAR). Established in 1990 as the Bioprocessing Technology Unit, it was renamed the Bioprocessing Technology Institute (BTI) in 2003. The research institute's mission is to develop manpower capabilities and establish cutting-edge technologies relevant to the bioprocessing community. Some of the key research areas include expression engineering, animal cell technology, stem cell research, microbial fermentation, downstream purification and analytics. For more information about BTI, please visit http://bti.a-star.edu.sg.
Contact:
Vanessa Loh
Senior Officer, Corporate Communications
Agency for Science, Technology and Research
Tel: +65 6826 6395
Email: vanessa_loh@a-star.edu.sg
Topic: Research and development
Source: A*STAR
Sectors: Science & Research
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|
|

|
|
|
|